doi.org/10.20986/resed.2024.4076/2023
NOTA CLÍNICA
Tolerance to intrathecal baclofen: clinical case report
Tolerancia a baclofeno intratecal. A propósito de un caso clínico
Claudia de la Fuente Escudero1
Jesús Benito-Penalva2
Joan Vidal Samsó2
Fernando García Pérez1
Ángel Antonio Verde Ortiz1
1Specialist Doctor in Physical Medicine and Rehabilitation. Hospital Fundación Alcorcón, Madrid, Spain
2Specialist Doctor in Physical Medicine and Rehabilitation. Neurorehabilitation Hospital Institut Guttmann. Badalona, Spain
ABSTRACT
Introduction: Widespread severe spasticity, resulting from a lesion or central nervous system disorder, which remains unresponsive to maximal oral drug doses or other techniques, may warrant consideration of surgical implantation of an intrathecal baclofen infusion system.
Materials and methods: This study entails a description of a clinical case along with a literature review.
Results: We present the case of a 46-year-old patient treated with an intrathecal baclofen pump for widespread severe spasticity secondary to an incomplete spinal cord injury, classified as C3 AIS C. Due to suspected tolerance development, a gradual tapering of intrathecal baclofen was initiated until complete withdrawal. Subsequently, the patient underwent a three-week morphine sulfate infusion before baclofen reintroduction. Follow-up at one and four months revealed sustained clinical improvement in spasticity with low doses of intrathecal baclofen.
Conclusion: The intrathecal baclofen pump ensures infusion of significantly lower baclofen quantities compared to oral ingestion. Catheter-related failure is the most common complication. However, in 5.9 % of prolonged treatment cases, a tolerance pattern has been documented, necessitating escalating intrathecal drug doses to sustain clinical efficacy. In such instances, a temporary cessation of baclofen, known as a “baclofen holiday”, may be considered.
Keywords: Intrathecal drug infusion, baclofen, spasticity, spinal cord injury, case report
RESUMEN
Introducción: La espasticidad severa generalizada, resultado de una lesión o trastorno del sistema nervioso central, que permanece sin respuesta a dosis máximas de fármacos orales u otras técnicas, puede justificar la consideración de la implantación quirúrgica de un sistema de infusión intratecal de baclofeno.
Materiales y métodos: Este estudio conlleva la descripción de un caso clínico junto con una revisión bibliográfica.
Resultados: Presentamos el caso de un paciente de 46 años tratado con una bomba de baclofeno intratecal por espasticidad severa generalizada secundaria a una lesión medular incompleta, clasificada como C3 AIS C. Debido a la sospecha de desarrollo de tolerancia, se inició una disminución gradual del baclofeno intratecal hasta su retirada completa. Posteriormente, el paciente fue sometido a una infusión de sulfato de morfina durante tres semanas antes de la reintroducción del baclofeno. El seguimiento al mes y a los cuatro meses reveló una mejoría clínica sostenida de la espasticidad con dosis bajas de baclofeno intratecal.
Conclusiones: La bomba de baclofeno intratecal asegura la infusión de cantidades de baclofeno significativamente menores en comparación con la ingestión oral. El fallo relacionado con el catéter es la complicación más frecuente. Sin embargo, en el 5,9 % de los casos de tratamiento prolongado se ha documentado un patrón de tolerancia que hace necesario aumentar las dosis de fármaco intratecal para mantener la eficacia clínica. En tales casos, se puede considerar una interrupción temporal del baclofeno, conocida como “vacaciones de baclofeno”.
Palabras clave: Infusión intratecal de fármacos, baclofeno, espasticidad, lesión medular, informe de un caso
INTRODUCTION
Spasticity contributes significantly to the disability of patients with brain damage or spinal cord injury (1). In cases where it does not respond to oral drug treatment or such medication causes side effects, long-term infusion of intrathecal baclofen may be considered by implantation of a programmable device in the abdomen (2).
Before undergoing an intrathecal baclofen infusion pump implant, a trial is commonly conducted to ensure the suitability and safety of the procedure for the patient. Typically, a preliminary dose of 50 to 100 μg is administered through lumbar puncture. A continuous trial involving a temporary catheter can also serve as a beneficial approach to gradually adjust the dosage for a more accurate evaluation.
Khurana et al, estimate that 1 in 3 patients will develop spasticity in the following 5 years after spinal cord injury and 20 % will have functional limitations secondary to this process. The 25-30 % of patients with spinal cord injury or multiple sclerosis will be non-responders to baclofen orally and approximately 5-10% of patients who have suffered a spinal cord injury will require treatment with intrathecal infusion (1).
Currently, there are only three drugs approved by the FDA (U.S. Food and Drug Administration) for intrathecal use. These are: morphine, ziconotide and baclofen (3).
Baclofen is a muscle relaxant analogous to the inhibitory neurotransmitter gamma-aminobutyric acid (GABA). being the drug of choice for the treatment of intrathecal spasticity (4).
It is described that up to 20 % of the cases (5) do not reach the therapeutic objectives despite the high doses of intrathecal baclofen. Although the dose is progressively increased up to 1000 mcg/day, after 18 months, the same clinical benefit that appeared at the beginning is not achieved. It is not common, but this process is known as baclofen tolerance (5,6,7).
Diagnosis of tolerance is difficult (8). Its prevalence is estimated at 5.9 % of cases (6). Initially, the lack of effect of intrathecal baclofen in a patient carrying a baclofen pump, a malfunction of the system should be suspected, most often being a complication secondary to the intradural catheter and more rarely to an intrathecal pump failure (3,5) to 78 % of cases.
The most recommended strategy to treat this problem currently is the temporary withdrawal of intrathecal infusion of baclofen, known as “drug holiday” or “baclofen holiday”, and its replacement with another drug, such as morphine (8).
CASE REPORT DISCUSSION
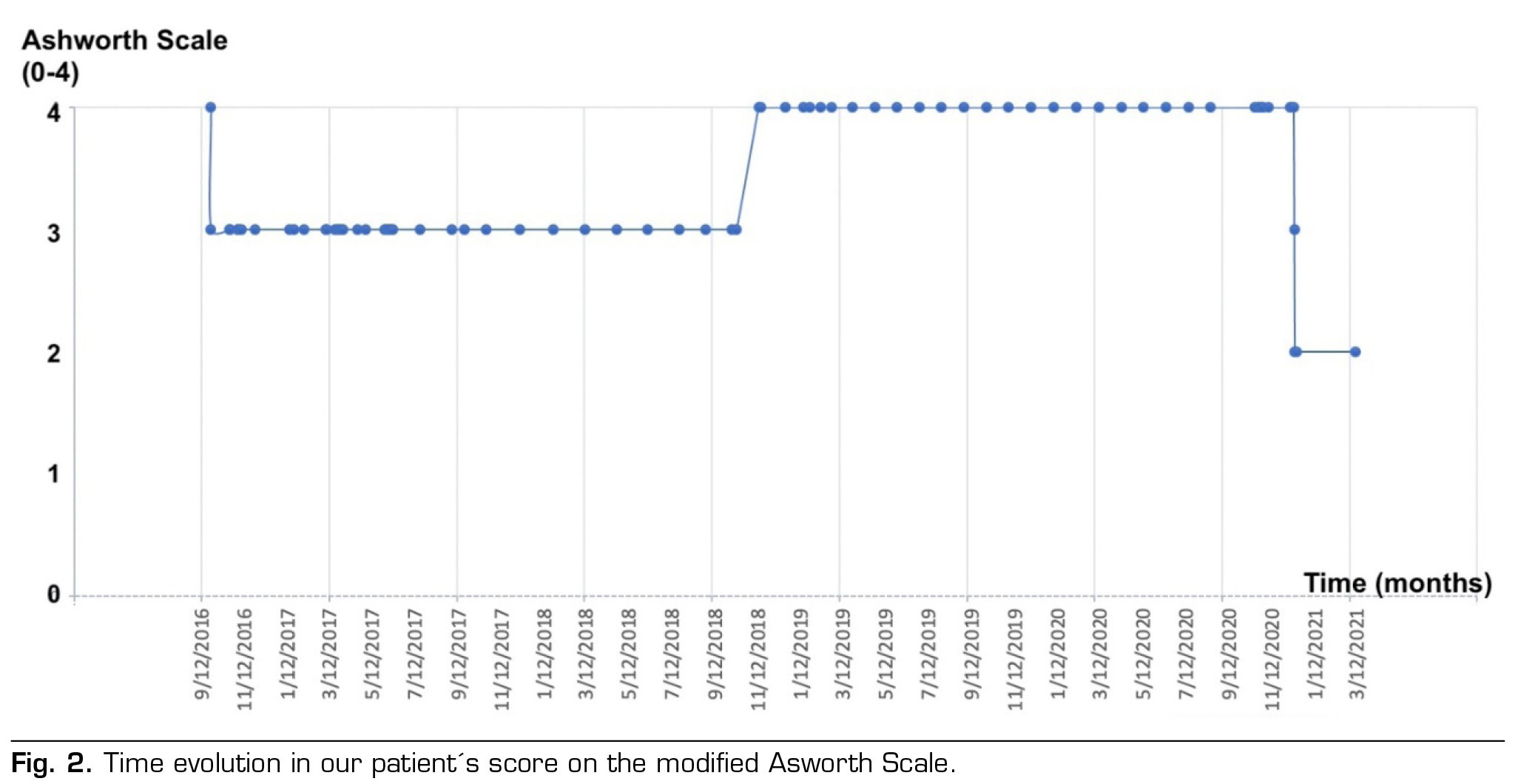
We present the clinical case of a 42-year-old male patient with after-effects of spinal cord injury with incomplete quadriplegia C3 AIS C (9), secondary to C3-C4 fracture when suffering a fall on a bicycle on August 7, 2013. The patient presented significant neuropathic pain associated with severe spasticity in all four extremities secondary to spinal cord injury. At 3 months after the lesion was established, a score of 2 in flexor and extensor elbow muscles and 3 overall in the rest of the muscles was recorded, according to the modified Ashworth scale (MAS) (1).
Initially the patient continued treatment with oral baclofen, at doses of 30 mg every 8 hours and tizanidine 4 mg every 8 hours, with clinical persistence.
In 2016, spasticity seriously interfered with sitting, hygiene, basic activities of daily living and night rest despite high doses of drugs (baclofen 35 mg every 8 hours and tizanidine 8 mg every 8 hours). It was decided to perform an intrathecal baclofen test with external pump. A significant reduction in spasticity was observed compared to an initial MAS value of 4, which decreased to 2, so it was decided to implant a continuous infusion system of intrathecal baclofen (Medtronic SynchroMed II), to improve the patient’s quality of life and comfort.
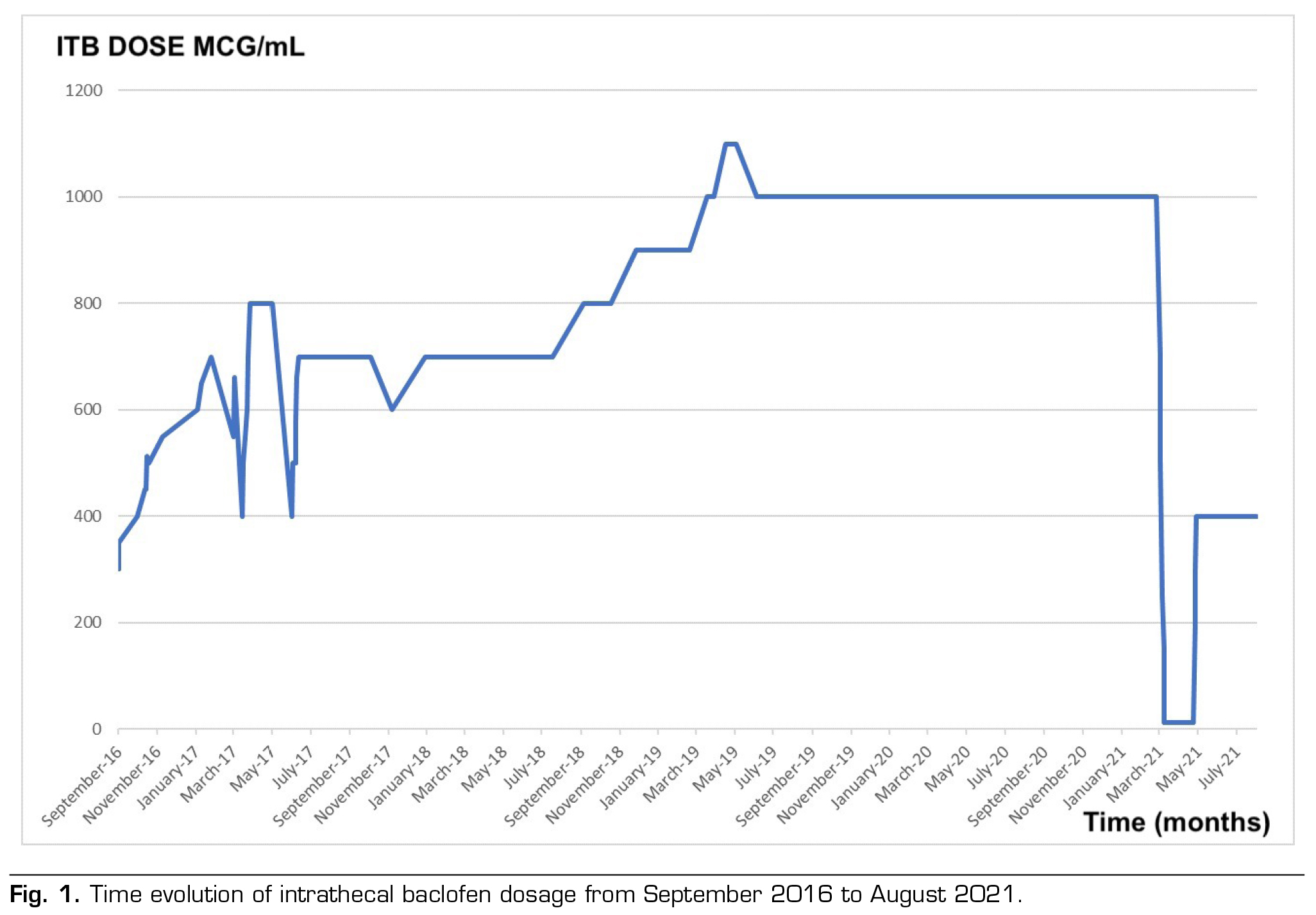
After implantation, the treatment was effective despite making walking difficult. The initial daily dose of baclofen was set at 400 mcg/day, which corresponds to double the effective bolus detection dose, without any negative side-effects. Within 7 months of the intervention, the clinic of severe spasticity reappeared, with no response to the progressive increase in dose or the change in the programming of the system. Likewise, the pump was emptied, noting that the actual volume of the tank corresponded to the theoretical one shown by the programmer, in addition to checking the cerebrospinal fluid that was clear and transparent. Considering the suspected malfunction of the catheter (Model 8731SC), a contrast myelography was conducted using the pump. This procedure revealed no signs of leaks. Furthermore, we ensured the contrast’s proper arrival within the upper dorsal level of the subarachnoid space. As there was a minor enhancement observed through bolus dosing, the programmer settings were retained accordingly. It’s worth noting that the procedure was executed with stringent safety protocols in place.
In 2017, the patient presents a new increase in spasticity. He reports difficulty in sitting and handling the electric wheelchair due to severe spasticity. At this time, a bolus administration Schedule was maintained every 4 hours at a total dose of 600 mcg/day. Given the new possibility of system malfunction and suspected catheter failure, a new transcatheter myelography was performed suggesting a rupture of the catheter, whose change was recommended taking place in May 2017.
Following the catheter replacement (using the same model as before), the pump was programmed to deliver boluses every 4 hours, accumulating a total daily dose of 700 mcg. This dose was progressively escalated until it reached a continuous mode administration of 1000 mcg/day. In the absence of a favorable response, a decision was made to alter the programming to a flexible mode, involving a total daily dose of 1000 mcg at a concentration of 2000 mcg/ml (comprising 13 boluses of 50 mcg each and a basal dose of 350 mcg/day).
Even with the administration of high doses, the challenge of managing spasticity continues, resulting in an overall MAS score of 3. Given the confirmation of the catheter’s proper functionality, the decision is made to conduct an intrathecal baclofen test through the administration of a 100 mcg baclofen bolus. This approach aims to differentiate between potential issues within the infusion system (such as the pump or catheter) and the possibility of developing tolerance to baclofen if no positive response is observed.
In March 2021, the patient is admitted to perform an intrathecal test through lumbar puncture, not obtaining a satisfactory response. Given the lack of efficacy and after ruling out other complications that could justify an increased in spasticity or malfunction of the infusion system, it is oriented as a possible tolerance to baclofen. It is decided to progressively reduce the infusion of intrathecal baclofen by 300 mcg every 3 days until its total suspension, and then initiating the infusion of morphine-sulphate through the continuous single mode infusion system. The use of intrathecal drug combinations was not considered as an option in this case due to a lack of safety and efficacy data.
During his stay, a good response to intrathecal morphine was verified. The treatment began with a dose of 1 mg/day at a concentration of 10 mg/ml, equivalent to 4 doses at a concentration of 0.25 mg/ml of intrathecal morphine and was gradually increased to 0,3mg/ml. This led to a reduction not only in the severe neuropathic pain he was experiencing, but also in the spasticity score as measured by the MAS scale. There were no observed side effects.
Subsequently, an outpatient control was carried out, after three and a half weeks with intrathecal morphine- sulphate. It is decided to perform the reintroduction of baclofen by reducing morphine 1 mg every 2 days until its withdrawal and start the baclofen infusion of 200 mcg/day until reaching 400 mcg/day, in continuous single infusion mode.
Currently the patient maintains a 1-2 in MAS score with doses of 400 mcg/day in continuous single mode allowing him to perform flexion and extension of limbs as well as assist in transfers.
Intrathecal therapy has been a great advance, since it allows to release drugs directly into the cerebrospinal fluid through which, the drugs reach the place of action, bypassing the first-pass metabolism and the filter of the blood-brain barrier (3).
In the literature reviewed, patients with prolonged treatment of baclofen may have complications related to device malfunction, mainly due to migration, rupture or disconnection of the catheter (6).
In cases where a limited response to therapy is observed, despite a progressive escalation and/or when high doses of the drug are reached without clinical benefit, a bolus regimen is recommended (3,10).
If, despite this, the patient continues to experience an increase in spasticity and requires higher doses of baclofen to maintain the previous clinical results, a system malfunction should be suspected, and if ruled out, baclofen tolerance.
Our clinical case concurs with the literature, in which at 24 months of implantation of the pump the dose is stabilized without clinical benefits (as shown in Figure 1). Additionally, Figure 2 shows the evolution of spasticity over time.

To make this diagnosis it is necessary to previously rule out a malfunction of the system by contrast myelography and also to check an absence of response to intrathecal baclofen by direct lumbar puncture (11).
As with oral baclofen, abrupt withdrawal of intrathecal baclofen can lead to serious complications, such as increased spasticity, hypertension, autonomic dysreflexia, and seizures. It should be phased out and may be replaced by another spasmolytic drug for days or weeks, until intrathecal baclofen therapy is restarted. The most commonly used drug in these cases is intrathecal morphine-sulfate (8,10,11) and can be considered appropriate during the “baclofen holiday” due to its antispastic effect. Despite not being approved by the FDA for the treatment of severe spasticity and its use for this application is considered off-label.
The current clinical management is based on clinical experience, there are no guidelines so future studies will be needed. As previously mentioned, intrathecal baclofen should be slowly decreased before starting with intrathecal morphine-sulfate. The reduction in our case was 300 mcg every 3 days and when it was at a dose of 300 mcg/day was when we introduced intrathecal morphine. Starting doses of intrathecal morphine range from 0.25 to 0.4 mg/ml (8).
The amount of time a patient stays with intrathecal morphine-sulfate may vary. There are studies that describe an average of 4 to 8 weeks. Patients may usually continue with intrathecal morphine-sulfate until they experience side effects. Most frequently described are itching, nausea and vomiting and urine retentions, which occur in 25 % of cases or worsening of spasticity despite increased morphine-sulfate doses. In these situations, intrathecal baclofen can be restarted (4,10,12).
It should be restarted at a low dose, as an overdose could occur, and increase progressively as done during a first implant. In addition, it may be necessary to supplement with oral antispasmodic drugs during this period to relieve increased of spasms (7).
CONCLUSION
The utilization of the intrathecal baclofen pump offers the advantage of delivering markedly reduced amounts of baclofen compared to oral administration. A tolerance phenomenon has been observed, which involves the need for progressively higher doses of intrathecal medication to sustain the intended therapeutic effect. In response to such circumstances, a potential strategy to address this tolerance is the temporary discontinuation of baclofen, a concept commonly referred to as a “baclofen holiday”.
Furthermore, it is important to highlight that, in specific cases, the substitution of baclofen with morphine- sulfate has been explored as an alternative antispasmodic treatment. This transition aims to enhance joint mobility and alleviate pain. Such an approach underscores the dynamic nature of therapeutic interventions in managing spasticity-related complications.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES