
DOI: 10.20986/resed.2019.3733/2019
REVIEW
Pain in Parkinson's disease. A look at a poorly known aspect of this disease
J. Rotondo1, M. Toro1, M. Bolívar1,2, M. E. Seijas1 y C. Carrillo1
1Anestesiólogo. Medicina Intervencionista del Dolor. 2Unidad de Medicina del Dolor (UMD) del Instituto Médico la Floresta. Caracas. Venezuela. Expresidente AVED.
Received: 21-08-2018
Accepted: 05-04-2019
Correspondence: Julio Rotondo
jcrotondo2005@gmail.com
ABSTRACT
Parkinson's disease (PD) is a neurodegenerative disease, the second most prevalent, after Alzheimer's disease. It presents both motor and non-motor symptoms; These include autonomic dysfunction, unexplained pain, cognitive impairment, anxiety, depression, among others. Some of these patients experience pain as an early symptom of Parkinson's, even before the expression of their disease. Among people who have PD and who experience pain, they describe it as a worrisome symptom, being a cause of suffering and disability. However, despite this, pain in PD often remains undiagnosed and untreated. Therefore, it is important to understand that pain can be part of the Parkinson's experience and learn ways to manage it.
This paper reviews current data on possible mechanisms, classifications, evolution, potential risk factors and pain control in PD. The mechanism of pain in this situation is complex, and is influenced by different factors, which may be linked to pathological changes in the anatomical structures involved in nociceptive mechanisms.
Key words: Pain, Parkinson's disease, dystonia, akathisia, dopamine.
RESUMEN
La enfermedad de Parkinson (EP) es una enfermedad neurodegenerativa, la segunda con mayor prevalencia después de la enfermedad de Alzheimer. Presenta tanto síntomas motores como no motores; entre estos últimos se encuentran disfunción autonómica, dolor inexplicable, deterioro cognitivo, ansiedad, depresión, entre otros. Algunos de estos pacientes experimentan el dolor como un síntoma temprano de Parkinson, incluso antes de la expresión de su enfermedad. Entre las personas que tienen EP y que experimentan dolor, lo describen como un síntoma preocupante, siendo una causa de sufrimiento y de incapacidad. Sin embargo, a pesar de ello, el dolor en la EP a menudo permanece sin diagnóstico y sin tratamiento. Por tanto, es importante entender que el dolor puede ser parte de la experiencia del Parkinson y aprender las formas de manejarlo.
Este trabajo revisa datos actuales sobre posibles mecanismos, clasificaciones, evolución, factores de riesgo potenciales y control del dolor en la EP. El mecanismo del dolor en esta situación es complejo, y está influenciado por distintos factores, pudiendo estar vinculado a cambios patológicos en las estructuras anatómicas involucradas en mecanismos nociceptivos.
Palabras clave: Dolor, enfermedad de Parkinson, distonía, acatisia, dopamina.
INTRODUCTION
Parkinson’s disease (PD) is presented as a chronic, long-term, irreversible pathology with symptoms that worsen over time. PD is the second most frequent neurodegenerative disease after Alzheimer’s disease. In countries such as the United States, a prevalence of 350 per 100,000 inhabitants is estimated; it is approximately twice more frequent in males than in females (1,2).
The PD was first described in 1817 by the British neurologist James Parkinson, who initially called it “alteration of the motor system” (3). Currently, PD is defined as a progressive neurodegenerative condition that arises as a consequence of an alteration in the production of dopamine by the dopaminergic neurons of the substantia nigra (SN), which causes an imbalance in the extrapyramidal control of the motor system; it is included within the movement disorders (4,5).
Symptoms usually appear after the age of 60 and it is very rare before the age of 30, although there is a minority of patients with early or juvenile onset (6,7). The prevalence ranges from 1% in the population around 65 years of age, to 5% at 85 years of age (8). After 90 years old, the onset of the disease is uncommon (2,9).
Despite the research efforts, the causes that trigger this disease are still unknown. In the pathogenesis of PD, several mechanisms are proposed as the cause of the lesion of the group of neurons present in the SN, without there being a directly related cause to date. Among these mechanisms, a combination of genetic predisposition with environmental factors is proposed (4,5,9,10). The genetic factor, which initially was proposed as the only cause, is more associated with the atypical form of early onset of PD that represents a minority of cases (1,6), which supports the theory stating that more than one factor (besides the genetic) can be the cause of this disease (9,11). It has been demonstrated with the identification of at least nine loci and the cloning of genes involved in the familiar form (11).
The oxidation triggered by the presence of free radicals (Table I), the action of exogenous toxins such as manganese and organophosphorus pesticides are among the proposed environmental factors; other still unknown environmental factors have been considered (7,9); thus, acute exposure to 1-methyl-4-phenyl-1,2,3,6 tetrahydropyridine, a toxic analogue to meperidine that is commonly found as a contaminant in illicit psychostimulants, is a known cause of rapid development of the PD and it has been used to obtain experimental models for their study.
This neurodegenerative disease is characterized by the presence of tremor at rest, bradykinesia or hypokinesia, unstable gait and muscle rigidity, together with other manifestations such as the alteration of postural reflexes, flexion posture and the phenomenon of freezing. To diagnose the disease, a patient should present at least two of the symptoms above mentioned, being essential the presence of tremor or bradykinesia. Other frequent findings are unexplained pain, constipation, urinary retention, sexual dysfunction, dementia, depression, altered sleep patterns or weight loss (9,12,13). They can be detected some years before the onset of any of the cardinal motor signs (14). A landmark study showed that virtually all PD patients report at least one nonmotor symptom when these manifestations are actively investigated using specific questionnaires, reaching an average of eight different symptoms per patient. In this study, the most frequent nonmotor symptoms were neuropsychiatric, gastrointestinal and pain-related symptoms (15). Others report that, in the early stages of the disease, the most common nonmotor symptoms are hyposmia, pain and sleep disorders (16).
Although pain has never been considered a primary symptom of PD, several researchers, including Charcot (17), had already referred to painful aspects of the disease: Sometimes Parkinson’s is preceded by neuralgic or rheumatoid pains, which are occasionally of great intensity, being located in the extremity (...) that will soon be affected (...) by the convulsive agitation.
NEUROANATOMIC AND NEUROPHYSIOLOGICAL CONSIDERATIONS OF PARKINSON’S DISEASE. NEUROCHEMICAL SCHEME OF THE BASAL GANGLIA CIRCUIT
Normally there is a balance between the contractile activity caused by acetylcholine and the inhibitory activity mediated by dopamine, which allows adequate tone and muscle function. There are five types of dopamine receptors: D1, D2, D3, D4 and D5. The types D1 and D5 are excitatory, whereas the remaining receptors are inhibitory, which means that the net effect of dopamine is the inhibition of cell action. By binding to its receptor, dopamine activates the G protein coupled to it. This causes the alpha subunits to separate from the rest of the protein and activates the adenyl cyclase that manages to increase the levels of cyclic AMP. Finally, there is an inhibitory effect on the action of the cell which, in the case of the muscle cell, would inhibit the contraction. In PD, the production of dopamine is impaired, and prevails the contractile effect of acetylcholine, whose striatal concentration remains normal (18).
In a healthy individual, the generation of coordinated motor movements by the brain lies in a balance between the activation of voluntary movements or direct pathway and the action of the inhibitory pathway or indirect pathway. Neurotransmitter dopamine and its receptors are involved in both pathways. Thus, under normal conditions, dopamine type 1 receptors (D1) are involved, located in the internal globus pallidus (GPi) and in the reticular part of the SN, and dopaminergic neurons that project to the external globus pallidus (GPe), represented by D2 receptors, are also involved. In patients with PD, due to the presence of a dopaminergic degeneration, these pathways are affected, resulting in a hypoactivity in the direct pathway and a hyperactivation in the inhibitory pathway.
The dorsal striatum (caudate-putamen) receives excitatory stimuli, glutamatergic, from the cortical motor areas. The final feedback of the dorsal striatum to the cortex is conducted by the motor thalamus and it is directed directly toward the motor cortex, where the areas involved in the movement organization are located. More than 90% of striatal neurons are medium-sized GABAergic neurons projecting outside the striatum. A simplified model of the functioning of the basal ganglia comprises two circuits already mentioned: one Direct and one Indirect (Figure 1). The glutamatergic neurons of the motor cortex innervate two main types of striatal GABAergic neurons. One type of neurons co-expresses the neuropeptides dynorphin (dyn) and substance P (SP) and has D1 dopaminergic receptors. These neurons directly project the GPi and the SN (compacta and reticulata), with an inhibitory action on both (direct pathway). The other type of GABAergic neurons expresses enkephalin (enk) and D2 dopaminergic receptors. These neurons indirectly project the GPi and SN through the GPe and the subthalamic nucleus (STN). First, they send inhibitory stimuli to the GPe, which in turn exerts an inhibitory effect (GABA action) on the STN. The latter exerts glutamatergic excitatory effect on the GPe, GPi and SN (indirect pathway). The dopaminergic neurons of the SN pars compacta (SNc) modulate the striatal impulses. It seems that they exert an excitatory effect on striatal GABAergic neurons with D1 receptors and exert an inhibitory effect on GABAergic neurons with D2 receptors. The activity of these dopaminergic neurons is controlled by the GABAergic projections from the striatum and by the glutamatergic projections of the STN. The main exit route of the basal ganglia takes place from GABAergic neurons of GPi, which project to the motor thalamus. The mentioned model is simplified, and does not explain the role of a percentage (10%) of GABAergic neurons of the striatum that express both D1 and D2 receptors, as well as the role of the cholinergic interneurons of the striatum, which also express somatostatin (19,20).
PATHOPHYSIOLOGY OF PARKINSON’S DISEASE
PD is characterized by the progressive loss of dopaminergic neurons in certain regions of the central nervous system, such as in the SNc, the locus coeruleus and raphe nuclei and neurons of the olfactory bulb; cholinergic, catecholaminergic and serotonergic neurons are also affected.
All this leads to an inability to perform the coordinated movements. The loss of dopaminergic communication between the basal ganglia and the striatum will generate a series of events that cause the classic symptomatology in patients with Parkinson’s disease.
The death of dopaminergic neurons in the SNc leads to the loss of dopamine in the caudate nucleus and putamen. The pathological manifestations include degenerative changes, such as neuronal death, depigmentation in the SN and the appearance of intracellular inclusions in dopaminergic neurons called Lewy bodies (see below). The functional effects of the loss of dopamine in patients with Parkinson’s can be understood according to the commented mechanism of the basal ganglia circuit (Figures 1, 2). Normally, dopamine exerts an inhibitory effect on GABAergic striatal neurons with D2 receptors (indirect pathway) and an excitatory effect on neurons with D1 receptors (direct pathway). By decreasing the inhibitory effect on neurons with D2 receptors, the GABAergic inhibitory action of the striatum on the GPe increases, slowing down the inhibitory action of GPe on the STN. This produces a release of the STN when this slowing down disappears, which increases the excitatory stimulus of the STN at all levels: SN, GPe and GPi. Furthermore, the decrease of the dopaminergic action on neurons with striatal D1 receptors leads to an attenuation of the GABAergic inhibitory action of the striatal neurons on the GPi. Therefore, as a final result, there is an increase in the GABAergic action of GPi in both direct and indirect pathways. This hyperactivity causes the inhibition of the motor thalamus that is responsible for the motor control of the cortical areas involved in the initiation of movements. The thalamus-cortex glutamatergic hypoactivity is related to symptoms such as rigidity, slowness of movement, akinesia, etc. The tremor is attributed to the “escape” of the motor thalamus from the powerful inhibition from the GPi, originating burst firing (every 125-250 ms) towards the cortex that are causing the muscular tremor (4-8 times per second) (21).
The appearance of parkinsonian symptoms does not occur until approximately 80% of the neurons in the SN have been lost (the cell count does not exceed 100 thousand) (22). The rest of the unaffected neurons present mechanisms of neuronal plasticity, producing an increase in the amount of dopamine and the development of hypersensitivity of dopaminergic receptors. Functional reserve through these adaptations explains the progression of PD, delaying the onset of the symptoms of the disease. Rapidly evolving lesions, such as those produced by neurotoxins, also produce parkinsonian symptoms. It has been postulated that some insult of toxic or viral type could accelerate the progressive neuronal degeneration. The anatomopathological studies of brains of post mortem patients who have suffered PD reveals the presence, already mentioned, of eosinophilic inclusions, called Lewy bodies, which contain proteins such as ubiquitin and alpha synuclein. The latter protein participates in the recycling of synaptic vesicles (23,24). These proteins accumulate forming “aggresomes”, which promote oxidative stress and apoptosis. These aggregates are not exclusive to PD and can be observed in patients with Alzheimer’s disease, Hallervorden-Spatz syndrome, and even in subjects without neurological disease (10).
Histopathology reveals the presence of dystrophic neurites throughout the central nervous system, as well as a variable loss of neurons in subcortical nuclei, particularly the SNc, the locus coeruleus, and the basal nucleus of Meynert and the dorsal motor nucleous of the vagus. Additionally, there is an intense depletion of melanized neurons (45-66%), as well as immunoreactive dopaminergic neurons for tyrosine hydroxylase (60-88%) of the SNc, particularly in the ventrolateral third (91-97%), which is projected to the striatum, followed by the mid-ventral, dorsal and lateral areas (25).


ENVIRONMENTAL FACTORS. ROLE OF OXIDATIVE STRESS
Although there is no direct evidence, different findings in humans and experimental animals support the hypothesis that oxidative stress is responsible for the death of dopaminergic cells in PD (Table I).
The dopaminergic neurons of the SN are more dependent on energy metabolism and mitochondrial oxidative phosphorylation than other brainstem populations (so they are more sensitive to oxidative stress); they also have low levels of calcium binding proteins, which implies a decrease in neuroprotective function.
As described, oxidative stress is the “driving force” of neurodegeneration and its cause is unknown (26). The evidence suggests that, in situations where antioxidant cellular defense fails to compensate for the increase in reactive oxygen species (ROS), it would be the prelude to cell death.
One of the possible explanations is that an increase in the accumulation of iron (cation) has been found inside the mentioned neurons of PD patients. The concentrations of iron are increased by 129% (27,28), whereas the concentrations of the antioxidant peptide glutathione are reduced by 40% (29), which could facilitate the appearance of reactions with the formation of strongly oxidizing radicals, such as superoxide ion. The nitric oxide synthetase enzyme is also increased in the glia of the SN (30), which leads to the formation of peroxynitrites and hydroxyl radicals, highly oxidizing. Excessive formation of reactive oxygen and nitrogen species leads to damage to proteins, lipids, DNA and RNA. The levels of protein carbonyls, protein oxidation markers, are twice elevated in the SN of patients with PD (31). The levels of lipid hydroperoxides, indicators of lipid oxidation, are tenfold increased (32). Finally, 8-hydroxyguanine, an indicator of oxidative damage in RNA and DNA, is also increased (33). In addition to oxidative stress, there is a toxic neurodegenerative cycle characterized by mitochondrial dysfunction, glutamate-mediated excitotoxicity and inflammation in the SN. All this explains the rapid progression of PD once the symptomatology manifests.
Because dopamine participates as a neurotransmitter at the systemic level in other body functions, a decrease in the levels of dopamine can be associated with other clinical alterations affecting, for example, blood pressure and cardiac contraction or hormonal regulation of prolactin. Therefore, additional events may occur in patients with PD, such as central hypoventilation, marked orthostatic hypotension, myoclonus or urinary incontinence (26).

PAIN IN THE PD. IMPORTANCE OF THE PROBLEM
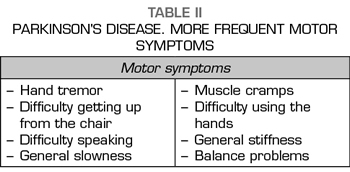
The interest in the study of motor symptoms in PD (Table II) has historically been larger than that shown by the assessment of nonmotor symptoms (Table III). Patients with PD may present with severe or untreatable pain, which may be more intense than motor symptoms, but still remains an underestimated symptom despite the fact that the correlation between pain and PD was described by James Parkinson as “ rheumatic pain that extends from the arms to the fingers “(34).
Although some studies place gradual importance to nonmotor symptoms, only a minority of studies have focused on the painful experience suffered by patients with PD.
Ability to diagnose and clinical experience to determine the cause of the pain are required. The most important diagnostic tool is the patient’s medical record.
Perhaps the most important task for people with PD experiencing pain is to describe as accurately as they can if the drugs induce, exacerbate or alleviate their pain. More than half of people with PD declare that they have suffered painful symptoms, stiffness, numbness and tingling at some point in the course of their illness. Pain in PD is an early symptom and can precede motor symptoms by several years (14,35,36).
The prevalence of pain in PD may vary from 34% (37) to 83% (38), depending on methodological evaluations.
Pain occurs two or three times more frequently in patients with PD than in individuals of similar age without PD (38-40).
Pain does not seem to be influenced by sex, age or geographic/cultural variables (41).
Pain usually occurs on the side where the motor symptoms appear or are more severe (42). However, this is not always the case, as some patients may have pain on the side not affected by the disease (43).
In terms of pain localization, although it can be quite variable, the lower back and legs are the most affected regions (41). Pain in the shoulder, commonly reported by patients with PD (15,43), may be the first symptom in 2-8% and may even precede the onset of motor symptoms (44).
Pain is believed to come from two different mechanisms: one mechanism directly related to the neurological symptom (pseudorheumatic and dopamine-sensitive), and the other mechanism could be associated with degenerative lesions that may worsen with the progression of PD.
People with pain and PD have higher scores on depression assessment scales (45). Therefore, it is important that in any assessment of the pain of an individual with PD, the possibility of the contribution of depression, which could require treatment, should be taken into account.
Another element to take into account are cognitive disorders, which can influence the perception of pain felt by the patient.
Systemic diseases, such as diabetes mellitus, osteoporosis and rheumatic diseases, are also associated with a higher prevalence of pain in PD (46). Finally, there is also evidence of an association between genetic factors and musculoskeletal pain; for example, mutations in the SCN9A (sodium channel Nav1.7) and FAAH (fatty acid amide hydrolase, a cannabinoid metabolizing enzyme) genes have been associated with an increased susceptibility to this symptom in PD (47).

ASSESSMENT AND DIAGNOSIS OF PAIN IN PATIENTS WITH PD
There is great variability in the characteristics of pain in PD, which can be mild to severe intensity, and of nociceptive and/or neuropathic components.
Lee et al. showed that 41.5% of patients in their series with pain and PD did not receive any analgesic treatment (48-50).
Among the diagnostic tools for assessing pain in PD, the most commonly used are: Brief Pain Inventory (36,38,51-56) and the Short Form (SF) 36 (49,57). The McGil pain questionnaire (38,58-60) is also very useful. More specifically, the pain of PD can be assessed through the DoPaMiP Study (Douleur et maladie de Parkinson in Midi-Pyrénées), which suggests that an initial distinction can be made between pain related to PD (PRPD) and pain not related to PD (PUPD) (38).
“King’s Parkinson’s disease pain scale”, which was proposed by a multicenter group at King’s College Hospital in London, is officially defended by the “International Parkinson and Movement Disorder Society Non-Motor PD Study Group” to evaluate the pain in the PD. It is a questionnaire with fourteen questions covering seven domains: 1) musculoskeletal pain; 2) chronic pain; 3) pain related to fluctuation; 4) night pain; 5) orofacial pain; 6) discoloration and edema/swelling; and 7) radicular pain (61). This is a new approach to pain in PD, which will allow more in-depth testing in clinical trials for treatments of this aspect of PD.
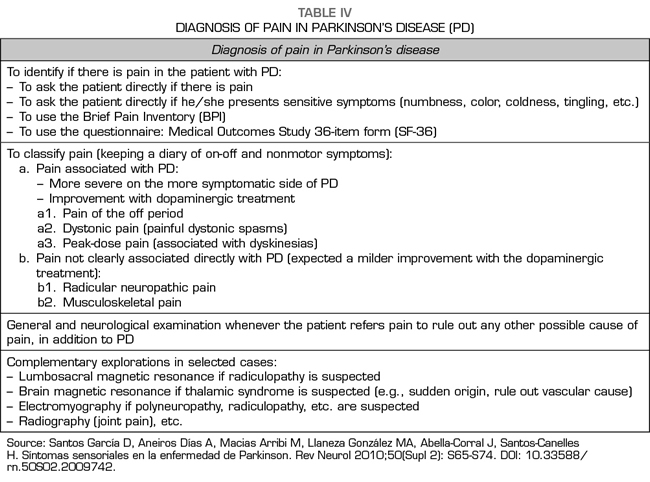
As above mentioned, pain may be the first symptom of the disease or it may present in advanced stages (62,63). The two keys that help to recognize that a pain is related to the PD itself are: its presentation or its maximum intensity on the side most affected by PD and that improves after the administration of dopaminergic medication. The characteristic is that the pain is present in “off” periods and pain improves during the “on” periods (64). Of course, a thorough general and neurological examination should be performed to rule out other pathologies.
The need or not to request complementary tests to determine the origin of the pain must be personalized in relation to the data obtained in the case history and in the physical examination of the patients. If the pain is lumbar and radiates to the legs, alterations of the sensitivity, force or reflexes are observed in the exploration, it would be necessary to rule out the presence of herniated discs or stenosis of the lumbar canal, so frequent in this age group. Sometimes, the pain is localized in the joints, and if it does not respond to the dopaminergic medication it would be necessary to perform imaging tests of the joint.
In the case of neuralgic pain, or if it has characteristics of polyneuropathy, an EMG should be requested if the pain persists with dopaminergic medication. If the pain has characteristics of thalamic pain, a brain MRI would be indicated.
The request for complementary tests in patients with pain and PD should be occasional if the patient clearly improves with dopaminergic medication.
For a systematic approach to diagnosis, please, see Table IV.
Quinn et al. (64) classified PA pain into four categories:
1. Pain that precedes the diagnosis of PD.
2. Pain of the “off” period.
3. Painful dystonic spasms.
4. Peak-dose pain.
For other authors, painful and disturbing syndromes in PD usually arise from these five causes, following the classification proposed by Ford (65.66), who was based on the studies of Goetz and Quinn (64.67) and that it implies the etiology of pain and its association with motor symptoms (Table II):
1. Musculoskeletal pain related to poor posture, inadequate mechanical function or physical wear.
2. Root pain, usually related to arthritis in the neck or back.
3. Pain due to dystonia, constant torsion of a group of muscles or part of the body in a forced posture.
4. Akathisic pain due to extreme restlessness.
5. A rare pain syndrome known as “primary” or “central” pain that arises from the brain.
Musculoskeletal pain
It is the most common and easily identifiable type of pain (36). Aching joints and muscles are particularly common in PD. Stiffness, lack of spontaneous movement, postural abnormalities and inadequate mechanical tensions in walking contribute to musculoskeletal pain (68).
With prolonged immobility of one of the limbs, band-like tendons may occasionally develop, usually in the hands or feet. An example is the clenched fist contracture of the hand, which can occur after a prolonged flexion of the fingers.
One of the most common musculoskeletal complaints is stiffness in the shoulders, associated with the intensity of akinesia, sometimes evolving into the so-called “frozen shoulder” (this, in fact, may be the first sign of PD) (69), related in turn to the increase in muscle inactivity. The prevalence of shoulder pain is estimated between 11 and 80%, whereas the prevalence of the frozen shoulder is between 2 and 8% in patients with PD (70,71).
In order to prevent shoulder problems, an assessment of range of motion in addition with a careful history of shoulder pain is critical to provide early diagnosis and treatment (72).
Occasionally, it can be challenging to distinguish between back pain due to PD and that caused by arthritis or scoliosis. Sometimes, other tests such as x-rays, ultrasounds, and rheumatologic or orthopedic examinations are required.
Gundogdu et al. (72) reveal that 58.9% of patients with PD have musculoskeletal pain, which was significantly higher compared to the control group. This is consistent with previous studies in which musculoskeletal pain was found in a range of 30%-70% pf patients with PD (73,74). This manifestation increases with age, affecting two thirds of patients with PD and with a higher prevalence of women (75-77). The most common musculoskeletal problem was low back pain (46.4%), in consistency with other studies (78,79).
Camptocormia in PD is a rare disease of muscle affectation, of late onset (80). Its symptomatology consists basically of chronic low back pain associated with a kyphosis secondary to weakness of the lumbar paravertebral musculature. Kyphosis increases with fatigue (81), is completely reducible in supine decubitus (82-84) and presents a female/male ratio of 4/1. In 20% of cases it is associated with a moderate deficit of the scapular or pelvic musculature.
The Pisa syndrome is a form of dystonic syndrome rarely described in PD and it is associated with striatal dopaminergic-cholinergic imbalance. It was first described in patients taking neuroleptic medication and characterized by a lateral tonic flexion accompanied by a rotation of the trunk in the sagittal plane (85,86).
The proper treatment for musculoskeletal pain in PD depends on the cause of the pain. If the pain is the result of excessive immobility or stiffening, the physician may indicate dopaminergic therapy, physiotherapy, and an exercise program that places strong emphasis on range of motion to prevent the development of musculoskeletal problems in the future. Nonsteroidal anti-inflammatory and analgesic drugs can help in orthopedic and rheumatological conditions.
Radicular and neuropathic pain
This affects from 5 to 14% of patients with PD with pain (38,42,49), and it is associated with focal compression associated with degenerative joint disease (68,87). Kyphosis and dystonia, which are clinical features of PD, can induce stress in the ventral portion of the lumbar disc, which leads to lumbar disc herniation and root pain (88).
A careful neurological evaluation is required to confirm the location of the nerve or nerve root involved, and thus determine the cause of the problem; Evaluations with electroneuromyography and neuroimaging may be necessary. The radicular pain is usually treated successfully with mobility and analgesic programs, so it rarely requires surgery. The first-line pharmacological treatments for neuropathic pain are amitriptyline, duloxetine and pregabalin (89).
Pain associated with dystonia
Dystonic spasms are among the most painful symptoms that a person with PD can experience. Dystonia is defined as a neurochemical-muscular alteration of the central nervous system (CNS) that leads to the appearance of involuntary muscle contractions that result in abnormal, painful, sustained and repetitive movements and/or postures that can affect different regions (90).
This type of muscle spasm is very different from the repetitive and oscillatory movements described as dyskinesia, which are not painful. Dystonia in PD can affect the extremities, trunk, neck, tongue, jaw, swallowing muscles, vocal cords, and lower extremities.
It is important to establish if painful dystonia is related to dopaminergic medication. Does dystonia occur when the medication is at its maximum effect? Or does dystonia appear as an “off” phenomenon when the effects of the medication start to fade at the end of the dose? The answers to these questions will, in general, clarify the nature and timing of the dystonia, and therefore it will determine its treatment. The most painful dystonia occurs when the decrease in plasma levels of the drug begins and commonly occurs early in the morning (68).
In terms of treatment, the dystonia of awakening is usually relieved by physical activity or with the first dose of dopaminergic medication. Few patients experience dystonic spasms as a result of medication. Standard treatment strategy for these individuals is to reduce the amount of dopamine, and sometimes replace dopamine with a less potent agent, or add medication for dystonia, such as amantadine.
Beiske et al. found that patients with dystonic and central pain after dopaminergic treatment had a better response (37% and 40% of cases, respectively) compared to patients presenting musculoskeletal and radicular neuropathic pain (17 and 14%, respectively) (50).
Individuals with an intractable dystonia may benefit from deep brain stimulation, a neurosurgical procedure that involves the implantation and activation of electrodes in the brain (68).
Central pain syndrome
It affects from 4 to 10% of patients with PD with pain. The pathophysiological mechanisms of this type of pain in patients with PD have not been well established (91). Although central neuropathic pain in PD was initially described by Souques (92) in 1921 as a primary parkinsonian pain, it is a symptom that has been little studied and it has been suggested that it could be caused by a dysfunction of the central nervous system in nociceptive processing (68,93), as suggested by the decrease in the threshold for pain produced by heat stimuli in some of these patients (94), as well as the alteration in the modulation of pain due to the dopaminergic deficiency in the basal ganglia-thalamus-cortex circuit (65).
The treatment of central pain in PD is challenging, and usually begins with the administration of dopaminergic agents (48,65,95). Additionally, treatment with opioids, antidepressants, anticonvulsants and antipsychotics may be useful in the treatment of central pain.
Akathisia
Akathisia is defined as a constant need to move or change position, which is a frequent and potentially disabling complaint, resulting in lack of sleep or social isolation. Although akathisia is sometimes described as a painful sensation, this is not usually the case and it should not be considered as a sensory disorder (36).
In approximately half of the cases of parkinsonian akathisia, symptoms fluctuate with medications and are usually relieved by additional dopaminergic treatment (72). If refractory to treatment, neurosurgical behavior is considered (see below), as well as deep brain stimulation and/or unilateral pallidotomy (96,97).
There are other painful syndromes that have been described in these patients.
Restless Legs Syndrome
Restless legs syndrome (98) (RLS) is common in patients with PD, and can be painful in some cases. It commonly occurs at the end of the day. It can be confused with akathisia. The RLS improves with dopaminergic medication.
Abdominal pain
It is frequent and may be related to constipation. The increase in the dosage of levodopa or dopamine agonists can worsen it. In other cases, patients in an “off” situation report a sensation of abdominal distension with flatulence accompanied by pain, which improves after levodopa administration (99).
PAIN CONTROL
Bearing in mind that there is a biochemical imbalance in which acetylcholine predominates over dopamine, pharmacological therapy is based on the administration of anticholinergic agents, dopamine substitutes, dopamine agonists, dopamine degradation blockers and adjuvant therapy (1,9,100).
The sensory phenomena occurring in states of dopaminergic hypofunction could be interpreted as a direct effect of the disinhibition of the sensory pathways from the ganglia of the base (64,101). Fil et al. (102) establish the following factors that potentially affect pain in PD:
– Advanced age: musculoskeletal pain is the most prevalent.
– Sex: Beiske et al. (50) found that the elderly woman is a predictor of pain in PD.
– Depression.
– Pre-existing diseases and systemic disorders.
Pain in PD is usually the result of inadequate dopaminergic therapy (103). People with PD who are in the “on” state, when the medication is at maximum efficacy, they report less pain than those in the “off” state (Table V). Pain due to dystonia or stiffness can be relieved with dopamine drugs, but on the other hand, it can lead to the development of dyskinesias. Therefore, effective management of levodopa medication for patients with PD can help to reduce pain.
When pain is very severe and we cannot suppress it during the off periods, we could try subcutaneous injections of apomorphine (104,105). In case of fluctuations that are not controlled using the usual medical treatment, the placement of an apomorphine pump or a continuous infusion of levodopa could be considered.
If the pain is associated with an “off” dystonia, it can be improved with local injections of botulinum toxin (106), applied to the muscles affected by dystonia.
Techniques such as cognitive-behavioral therapy (which helps to control the psychological response to pain, the teaching of diaphragmatic breathing, visual image exercises, relaxation techniques, etc.) and biofeedback have also proven useful.
Within some of these pharmacological pathways we have (18):
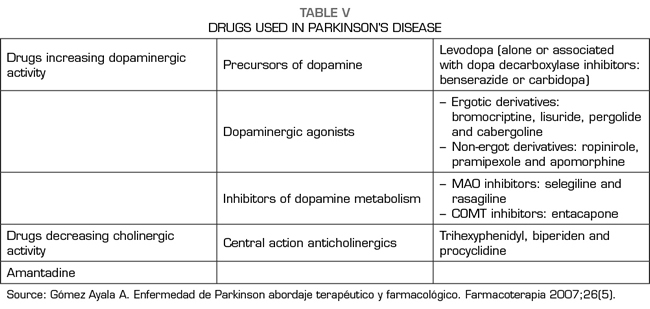

Anticholinergics
Anticholinergics are useful in the early stages for the control of stiffness and tremor, by restoring the balance between dopaminergic and acetylcholinergic activity. Procyclidine, isotazine, trihexyphenidyl, biperiden and ethopropazine are some anticholinergics. Adverse effects include mydriasis, dry mouth, constipation, urinary retention and psychiatric effects such as loss of memory and concentration, confusion and visual hallucinations. The abrupt suspension of anticholinergics could cause the exacerbation of parkinsonism and the precipitation of the cholinergic crisis.
Substitutes for dopamine
Levodopa is the most effective medication for the treatment of PD. Since dopamine does not cross the blood-brain barrier, its precursor, levodopa, is administered, which effectively increases the synthesis of dopamine in the neurons remaining in the nigrostriatal system. Given that the enzyme decarboxylase, that transforms levodopa into dopamine, is present at the central and peripheral levels, the administration of levodopa must be accompanied by a dopa-decarboxylase inhibitor that does not cross the blood-brain barrier, such as carbidopa or benserazide, in order of avoiding peripheral metabolism of levodopa. This therapeutic inhibition is important to increase the availability of levodopa at the central level and to reduce undesirable peripheral effects, such as the activation of the chemoreceptor region of the area postrema, which is located outside the blood-brain barrier and whose activation by dopamine causes important emesis.
It is usually used when symptoms of parkinsonism cause a certain degree of disability and, usually, its use is avoided at the beginning of the disease, since it usually causes dyskinesia and worsen other symptoms such as postural hypotension and psychiatric symptoms. Additionally, the patient may eventually develop a fluctuating response to therapy, with periods of relatively good control of symptoms and sudden onset of symptoms. This is known as an on-off phenomenon and can become really incapacitating given the unpredictability of its appearance.
Dopamine receptor agonists
They are usually used when the response to levodopa treatment declines or when it causes adverse effects. Among them are bromocriptine, pergolide and lisuride. Side effects include hypotension, nausea, vomiting and hallucinations.
Dopamine degradation blockers
Another option is the use of drugs that block the metabolism of dopamine by monoamine oxidase (MAO), such as selegiline (107).
COMT inhibitors
These drugs increase the brain values of levodopa by inhibiting its metabolism. Its use is, therefore, indicated in association with levodopa in patients with dopa therapy and motor fluctuations. In this sense, the “on” periods increase and the “off” periods decrease in patients with these fluctuations, in mild to moderate illness.
The fact that apparently COMT inhibitors also improves motor function in patients without fluctuations, makes some authors propose the use of COMT inhibitors associated with levodopa since the beginning of treatment, although this point is not yet clearly established. The use of COMT inhibitors, however, may increase the risk of dyskinesias.
Adjuvant medication
Amantadine is an antiviral agent whose mechanism of action is not completely clarified, but that probably acts facilitating the release of dopamine at the striatal level, blocking its reuptake and stimulating dopaminergic receptors (9). The use of amantadine should always be considered before deciding on the surgical treatment of dyskinesias secondary to chronic L-dopa therapy (108).
In addition, the use of other medications for the control of nonmotor symptoms associated with PD (depression, constipation, psychosis, etc.) must be taken into account. These include antidepressants (tricyclic antidepressants, serotonin reuptake inhibitors), laxatives or enemas, and antipsychotics such as clozapine and quetiapine (109).
If the response to pain is insufficient, tricyclic antidepressants could be useful (110), since they have an anticholinergic effect; the serotonin reuptake inhibitors exacerbate parkinsonism in some patients (7). Another measure would be to try antiepileptic drugs such as gabapentin or pregabalin, although there is no studies supporting their use.
Cannabinoids
A special mention deserves the cannabinoids, which can act, with neuroprotective efficacy, on some of the commented phenomena related to the nigrostriatal damage, which could be useful to diminish or slow the course of the disease. Both exogenous and endogenous cannabinoids (which include the classics, such as anandamide and 2-arachidonylglycerol, and cannabinoid analogues, such as oleoylethanolamide [OEA] and palmitoylethanolamide [PEA]) have neuroprotective efficacy. Cannabinoid analogues act primarily through peroxisome proliferator-activated receptors (PPAR), which include PPARalpha, PPARbeta, and PPARgamma (111).
Certain cannabinoids, such as cannabidiol, delta-9-THC, cannabinol, nabilone, etc., have the ability to reduce oxidative stress (112). Studies with animal models of PD confirm this fact, and their effects seem to be due to antioxidant properties per se, since they do not act through the CB1 or CB2 cannabinoid receptors (113). In cell cultures of dopamine neurons of the SN, delta-9-THC also shows antioxidant activity against the toxic 6-OHDA, without participation of CB1 receptors. The OEA and the PEA also have antioxidant properties, since the agonist action on PPAR decreases the oxidative stress induced by 6-OHDA (114), reduces the activity of the enzyme nitric oxide synthetase (iNOS), of great oxidative importance (115), and it increases the activity of cerebral antioxidant enzymes (116). These cannabinoid analogs are very interesting candidates as neuroprotective compounds because they are produced by the neurons themselves and the glia in response to oxidative stress (117,118). Several experiments have shown this efficacy, but also a possible “window” effect (only certain doses are effective) that could limit their use. This effect is due to the fact that they co-act on the TRPV1 receptor and the activation of this receptor has deleterious actions on dopaminergic neurons (119,120). Therefore, it is believed that these compounds have PPAR stimulating activity and TRPV1 blocking activity, which could be of greater neuroprotective efficacy.
Cannabinoid agonists possess anti-excitotoxic capacity, decreasing the release of glutamate, through CB1 presynaptic receptors (121,122). They can be effective in reducing excess glutamatergic activity from the STN, a hyperactive center in PD, as do other drugs such as riluzole, currently in clinical trials. CB1 cannabinoid agonists also have anti-inflammatory properties, since they reduce the levels of inflammatory cytokines, such as TNF-alpha and IL-12, or increase the levels of IL-10, an anti-inflammatory cytokine (123). PPAR agonists decrease the activity of proinflammatory factors, such as NFkB, AP-1 and NFAT.
In summary, various cannabinoids show neuroprotective efficacy on dopamine neurons, through an antioxidant, anti-inflammatory or anti-excitotoxic activity. The endogenous release of cannabinoids after neuronal damage constitutes a physiological protective response.
In extreme cases, and with absence or poor response to pharmacological therapy, the possibility of surgical treatment would be considered (124). Among these surgical options, there are the following:
– Pallidotomy: it injures the GPi and the ansa lenticularis, which relieves excessive thalamic inhibition and improves symptoms such as stiffness and bradykinesia.
– Thalamotomy: improves tremor.
– Deep subthalamic stimulation: through the implantation of electrodes, it can be directed to the GP or to the STN, achieving improvement of the contralateral hemibody tremor (125,126).
– Implantation of fetal cells in the SN: significantly improves symptoms by growing and forming new synapses in the transplanted tissue.
CONCLUSIONS
In recent years, nonmotor symptoms in PD have received increasing attention from physicians and researchers. Pain is present in the preclinical and initial stages of the disease and it is a cause of suffering and disability in patients with PD, causing a great impact on the quality of life and unfortunately, very often, pain goes unnoticed in clinical practice because usually the greatest attention is given to motor alterations. The prevalence of pain in patients with PD varies between 34% and 83%. It has been described that in the early stages of the disease, there is back and neck pain, which can result from stiffness in the shoulder girdle, and pain in the legs that can be a result of the syndrome of restless legs or dystonia. In advanced stages, pain can be caused by dyskinesia, akathisia, dystonia of the “off” period (40%) and nondystonic pain of skeletal muscle, articular or radicular type (20%), as described by James Parkinson in his original article on “paralysis agitans”. In general, pain in PD is usually the result of inadequate dopaminergic therapy, improving if a more continuous stimulation of dopaminergic receptors with levodopa is achieved, adding COMT inhibitors or dopamine agonists. Although the pain in PD is generalized and incapacitating in some cases, its clinical and pathophysiological characteristics have not been fully defined. Further studies are needed to clarify these points and to elucidate the role of dopaminergic and nondopaminergic pathways in nociception, central modulation of pain and affective/motivational dimensions in the perception of pain.
BIBLIOGRAPHY